The Task 5 Initiative
Identifying Metrics to Support Informed Decision-Making by Critical Audiences
The Latest
Meeting Report from 2022 Consensus Conference
Snyder JJ, Schaffhausen CR, Hart A, Axelrod DA, Dils D, Formica Jr RN, Gaber AO, Hunt HF, Jones J, Mohan S, Patzer RE, Pinney SP, Ratner LE, Slaker D, Stewart D, Stewart Lewis Z, Van Slyck S, Kasiske BL, Hirose R, Israni AK. Stakeholders’ Perspectives on Transplant Metrics: The 2022 Scientific Registry of Transplant Recipients’ Consensus Conference. Am J Transplant. 2023 Jul;23(7):875-890. doi: 10.1016/j.ajt.2023.03.012.
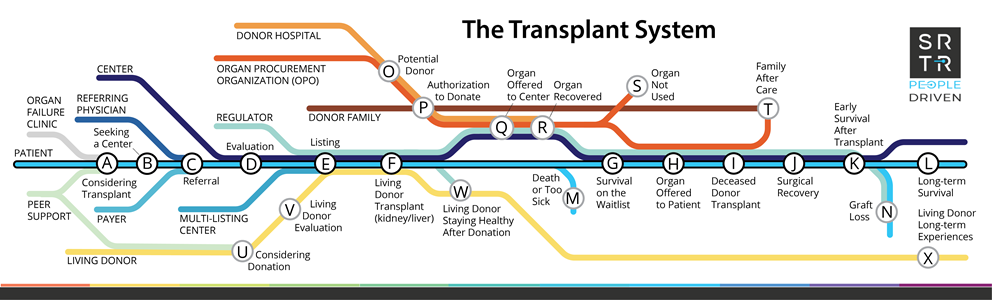
Updated from the transplant system map originally shown in the workbook for the SRTR People Driven Transplant Metrics Consensus Conference, Bloomington, Minnesota, July 18-20, 2022.
2022 Consensus Conference Videos
Watch the 2022 Consensus Conference On Demand videos on our YouTube channel:
About the 2022 Consensus Conference
The Scientific Registry of Transplant Recipients (SRTR) brought together representatives from various stakeholder groups to make recommendations for better metrics to support the field of organ donation and transplantation at the People Driven Transplant Metrics Consensus Conference. This conference was held on July 18-20, 2022, at the Radisson Blu hotel in Bloomington, Minnesota. SRTR continues to digest all the feedback received through the various sessions and breakouts. Progress updates continue to be provided periodically.
Conference materials included:
Agenda (Updated July, 2022)
Conference Program (Updated July, 2022)
Conference Workbook (Updated July, 2022)
As part of the Task 5 Initiative, SRTR spent a year collecting feedback from the transplant community about transplant care, access to information, and what changes community members would like to see:
The Scientific Registry of Transplant Recipients (SRTR) is operated under contract from the Health Resources and Services Administration (HRSA). While many tasks are required of SRTR, HRSA established "Task 5" in September 2020 with the goal to "identify metrics to assess national transplantation system performance and support informed decision-making by critical audiences." The three main goals of the task are:
- Identify critical audiences that need information to assess organ procurement, transplant, and the procurement and transplant system.
- Identify information of interest to critical audiences.
- Develop assessments and metrics that monitor information of interest to these critical audiences, in conformance with the reporting requirements of the OPTN Final Rule.
1. Identify Critical Audiences
On March 16, 2000, the US Department of Health and Human Services (HHS) published the Organ Procurement and Transplantation Network (OPTN) Final Rule [i], which established a regulatory framework for the operation of SRTR. The Final Rule requires SRTR to "make available to the public timely and accurate program-specific information on the performance of transplant programs." [ii] The "public" is made up of many stakeholders, including but not limited to:
- Patients with end-stage organ disease who are not yet listed for transplant
- Transplant candidates on the OPTN waitlist
- Family members or caregivers of patients and candidates
- Potential living donors
- Potential living donor organ transplant recipients
- Deceased donor families
- Transplant professionals
- Transplant programs and hospitals
- Organ procurement organizations
- The OPTN
- Third-party reimbursement entities
- The federal government
During the Steering Committee meeting on April 15, 2021, members discussed the general list of stakeholders above, refining it to include:
Patients and family members
- Transplant recipients
- Patients with late- or end-stage organ disease who are not yet listed for transplant
- Patients referred for transplant but not yet listed
- Patients listed for transplant
- Potential living donors
- Family members or caregivers of patients
- Deceased donor family members
Transplant professionals
- Medical professionals
- - Physicians
- - Surgeons
- - Nurses
- - Transplant coordinators
- - Quality Assurance and Performance Improvement (QAPI) staff
- - Transplant administrators
- - Transplant social workers
- - Transplant pharmacists
- Transplant programs/hospital
- Organ procurement organizations (OPOs)
- End-stage renal disease networks
- Professional societies:
- - American Society of Transplantation (AST), American Society of Transplant Surgeons (ASTS), Association of Organ Procurement Organizations (AOPO), North American Transplant Coordinators Organization (NATCO)
- - The Transplantation Society
- - American Society of Nephrology
- - The American Association for the study of Liver Disease
- - International Society for Heart and Lung Transplantation
- - American Nurses Association
- - Society for Transplant Social Workers
Government
- OPTN
- Executive branch
- HHS
- - Health Resources and Services Administration
- - Centers for Medicare & Medicaid Services
- - National Institutes of Health
- - US Food and Drug Administration
- - Centers for Disease Control and Prevention
- Legislative branch (Congress)
- Judicial branch (i.e., use of SRTR data in judicial matters)
- State government/legislators
Other
- Third-party payers
- Patient organizations
- - Transplant Recipients International Organization
- - National Kidney Foundation
- - American Association of Kidney Patients
- - American Liver Foundation
- - Juvenile Diabetes Research Foundation
- - Transplant families
- - Children's Organ Transplant Association
- - Starzl Network
- Allied organizations
- - UNOS
- - Donate Life America
- - Organ Donation and Transplantation Alliance
- - American Foundation for Donation and Transplantation
- Paired donation programs
- - National Kidney Registry
- - Alliance for Paired Donation
- Industry
- - Device manufacturers (e.g., ex-vivo perfusion manufacturers)
- - Pharmaceutical companies
- - Biotechnology companies
- Researchers
- Press
SRTR and the Steering Committee will continue to refine this list of potential stakeholders and welcomes feedback.
2. Identify Information of Interest
Once critical audiences have been identified, Task 5 requires SRTR to engage those audiences to "identify information of interest to critical audiences that need information to assess organ procurement, transplantation, and the procurement and transplantation system." SRTR has engaged the critical audiences in three primary ways.
 First, SRTR convened a consensus conference in summer 2022. The conference brought together stakeholder representatives to hear perspectives and make recommendations about data and metrics SRTR should provide to the community. The conference was held July 18 - 20, 2022 in the Minneapolis, Minnesota, area.
First, SRTR convened a consensus conference in summer 2022. The conference brought together stakeholder representatives to hear perspectives and make recommendations about data and metrics SRTR should provide to the community. The conference was held July 18 - 20, 2022 in the Minneapolis, Minnesota, area.

Second, SRTR convened patient and family focus groups before the July 2022 consensus conference. Focus groups were led by Allyson Hart, MD, SRTR senior staff for patient and family affairs. Dr. Hart worked with the Steering Committee to finalize plans for focus group recruitment and the discussion guide. The committee recommended that recruitment for focus groups ensures a broad range of perspectives from patients, including gender, racial, ethnic, and geographic diversity, and broad representation across organ types and adult and pediatric concerns. SRTR invited the public to nominate potential participants for these focus groups.
 Third, SRTR opened public comment to hear input from stakeholders. SRTR sought comment on which metrics would best meet each stakeholder's needs and recommendations for how those metrics should be constructed and presented.
Third, SRTR opened public comment to hear input from stakeholders. SRTR sought comment on which metrics would best meet each stakeholder's needs and recommendations for how those metrics should be constructed and presented.
3. Develop Assessments and Metrics
After the July 2022 consensus conference, SRTR started evaluating and implementing the recommendations. SRTR now continues to work with HRSA and the SRTR Review Committee and its subcommittees to implement the recommendations. The SRTR Review Committee currently has three subcommittees:
- Analytical Methods Subcommittee: Helps SRTR develop analytic methods to create any new metrics.
- Human Centered Design Subcommittee: Helps SRTR develop new data presentations, website changes, report changes, etc., to effectively disseminate the data in a manner that meets the needs of critical audiences.
- Patient and Family Affairs Subcommittee: Helps SRTR to effectively present data and metrics to meet the needs of patients and family members.
How can I stay informed?
Stay informed by subscribing to the SRTR Data Review newsletter and following SRTR on Twitter and LinkedIn.
SRTR has established the Task 5 Steering Committee to guide it in fulfilling the objectives established by HRSA. The Steering Committee is made up of the following members representing different stakeholders:
| Member | Stakeholders |
|---|---|
| Valerie Caldwell-Johnson, MSN | CMS-CCSQ |
| Dorrie Dils | AOPO representative |
| Shannon Dunne, JD; Chris McLaughlin | HRSA (ex-officio) |
| Tom Duvall, MBA | CMS-CMMI |
| Richard Formica, MD (backup: Sean Pinney, MD) | AST representative |
| A. Osama Gaber, MD (backup: Lloyd Ratner, MD, MPH) | ASTS representative |
| Heather Hunt, JD | Living donor |
| Jennifer Jones | Transplant patient |
| Zoe Stewart Lewis, MD, PhD | OPTN Membership and Professional Standards Committee |
| Sumit Mohan, MD, MPH | SRTR Review Committee |
| Rachel Patzer, PhD, MPH | OPTN Data Advisory Committee |
| Dirk Slaker, MD | Third-party payers representative |
| Sean Van Slyck | OPO executive |
Abbreviations: AOPO: Association of Organ Procurement Organizations; AST: American Society of Transplantation; ASTS: American Society of Transplant Surgeons; CCSQ: Center for Clinical Standards and Quality; CMMI: Center for Medicare and Medicaid Innovation; CMS: Centers for Medicare & Medicaid Services; OPO: organ procurement organization; OPTN: Organ Procurement and Transplantation Network; UNOS: United Network for Organ Sharing.
Task 5 will be accomplished over 5 years, from September 2020 through September 2025, with the work being broken down into four distinct phases:
- Phase 1. Planning: In 2021 and early 2022, SRTR convened the
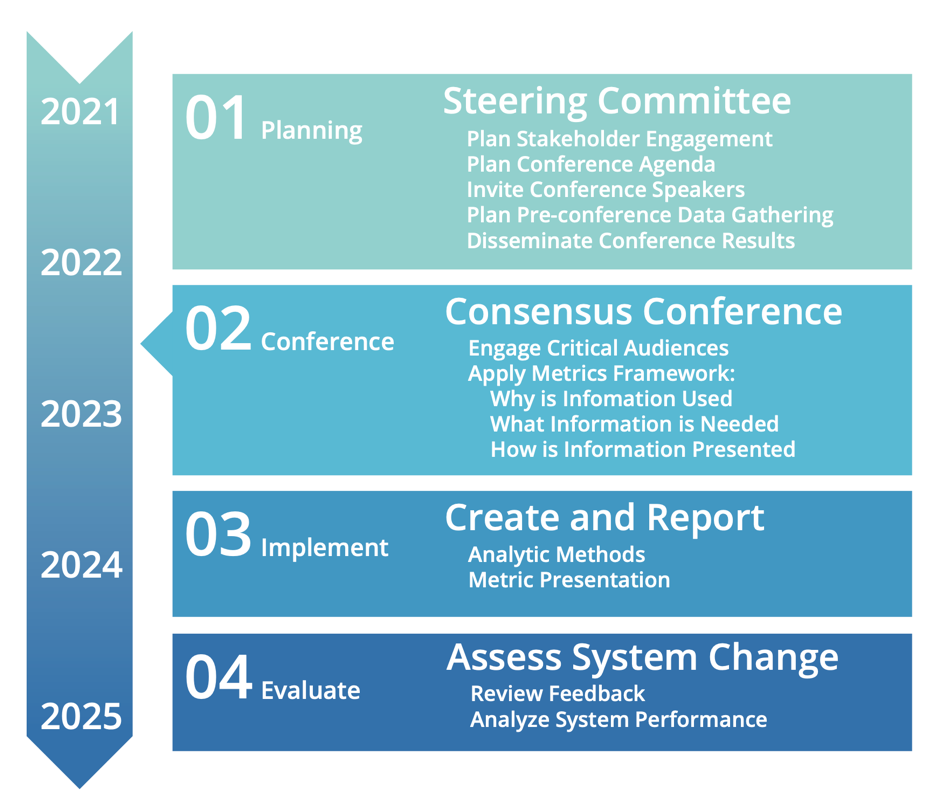 Steering Committee monthly to plan a successful consensus conference in summer 2022. Patient and family focus groups also met during phase 1.
Steering Committee monthly to plan a successful consensus conference in summer 2022. Patient and family focus groups also met during phase 1. - Phase 2. Consensus conference: In July 2022, SRTR engaged critical audiences in a consensus conference to develop concrete recommendations for metrics to meet the needs of critical audiences and understand the information needed to support those metrics and how the metrics should be presented.
- Phase 3. Create and Report: According to the recommendations of the consensus conference, SRTR developed and reported the recommended metrics (see the meeting report). If additional data collection is required to support any new metrics, SRTR will work with HRSA and the OPTN to strategize that collection.
- Phase 4. Assess System Change: In an ongoing effort to ensure
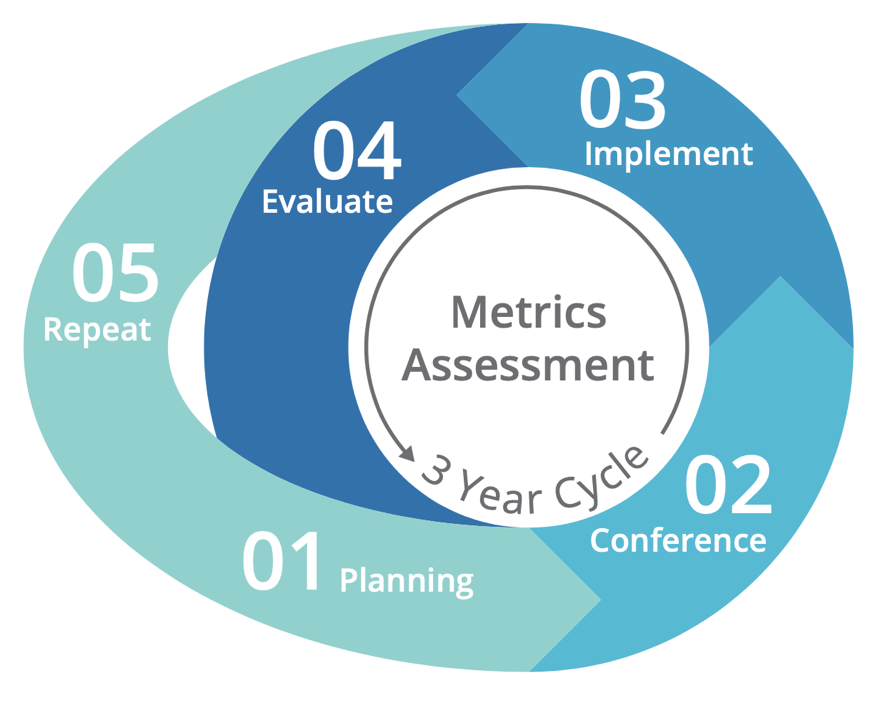 continuous evaluation and improvement, SRTR will review feedback and systematically assess the effectiveness of any changes to metrics. SRTR will convene stakeholders again in 2025 to assess the effectiveness of the changes. Going forward, SRTR will begin a 3-year cycle to engage stakeholders, implement recommendations, and evaluate the effectiveness of any changes.
continuous evaluation and improvement, SRTR will review feedback and systematically assess the effectiveness of any changes to metrics. SRTR will convene stakeholders again in 2025 to assess the effectiveness of the changes. Going forward, SRTR will begin a 3-year cycle to engage stakeholders, implement recommendations, and evaluate the effectiveness of any changes.
The following staff lead SRTR's Task 5 efforts and work with the Steering Committee to ensure the project's success:
| SRTR Staff | Role |
|---|---|
| Jon Snyder, PhD | SRTR Director |
| Ajay Israni, MD, MS | SRTR Deputy Director and Medical Director |
| Ryo Hirose, MD | SRTR Surgical Director |
| Allyson Hart, MD, MS | SRTR Senior Staff, Patient and Family Affairs |
| Dorry Segev, MD, PhD | SRTR Senior Staff, Special Studies |
| David Axelrod, MD, MBA | SRTR Senior Staff, Economics |
| Cory Schaffhausen, PhD | SRTR Human Centered Design Engineer |